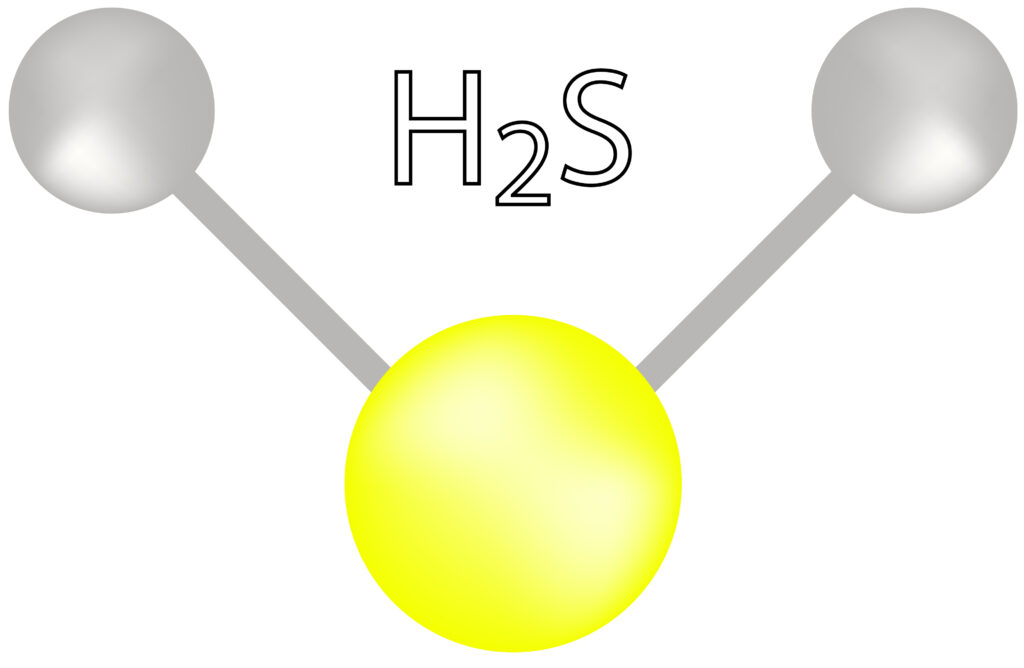
What is hydrogen sulfide?
Hydrogen sulfide (H2S) is a colorless, toxic, highly flammable gas with a pungent odor similar to that of rotten eggs. Often referred to as “sewer gas”, hydrogen sulfide is a result of the breakdown of organic matter by bacteria, typically in an oxygen deprived environment such as a municipal collection system or wastewater treatment plant.
Controlling this hazardous gas is one of the most challenging problems facing industry and wastewater treatment personnel today. Not only is it a nuisance because of its odor, hydrogen sulfide has the potential, even at low concentration levels, to pose extreme safety hazards for utility personnel, while its corrosive nature is detrimental to plant and collection system equipment. The concentration levels of hydrogen sulfide in most wastewater collection systems and sludge processing plants are dependent on several factors.
- Agitation – although agitation does not affect the amount of hydrogen sulfide produced, it does play a factor in how much H2S is released. The greater the agitation in a manhole or pump station, the greater amount of H2S that will be released into the air.
- Temperature – sulfate reducing bacteria thrive in the wastewater stream in elevated temperatures up to 98°F. Therefore, the higher the temperature of the wastewater, the greater concentration of sulfate reducing bacterium that produce hydrogen sulfide.
- Microorganism and Sulfate Counts – sulfates serve as an oxygen source for microorganisms in the wastewater stream. When sulfates are consumed, hydrogen sulfide is produced by the microorganisms as a by-product. Thus, the greater the sulfate levels in the wastewater stream, the greater concentration of sulfate reducing bacterium that produce H2S.
- Residence Time – everyday, miles of sewer lines are installed to accommodate economic expansion. As a result, sewer lines are often oversized to compensate for the rapid growth. These oversized sewer lines lead to greater wastewater residence times. High levels of H2S are produced during these residence times.
Dangers of Hydrogen Sulfide Exposure
Hydrogen sulfide is most commonly recognized by its distinct smell. However, prolonged low-level exposure and short term moderate level exposure will cause loss of smell even though the gas may still be present.
| Hydrogen Sulfide Concentration: | Side Effects: |
| Low Level: | fatigue, severe headache, irritation to eyes, nose, throat, or respiratory system |
| Moderate Level: | possible loss of consciousness, difficulty breathing, nausea, vomiting, dizziness, confusion, severe irritation to eyes, nose, throat, and respiratory system |
| High Level: | seizures, convulsions, inability to breathe, possible death |
Hydrogen Sulfide Toxicity Chart
| ppm of H2S: | Side Effects: |
| 10 ppm | Can smell. Safe for 8 hours exposure. |
| 100 ppm | Kills smell in 3-15 minutes. May sting eyes and throat. |
| 200 ppm | Kills smell quickly. Stings eyes and throat. |
| 500 ppm | Lose sense of reasoning and balance. Respiratory paralysis in 30-45 minutes. Needs prompt artificial resuscitation. Will become unconscious quickly (15 minutes max). |
| 700 ppm | Breathing will stop and death will result if not rescued promptly. Immediate artificial resuscitation is required. |
| 1000 ppm | Immediate unconsciousness. Permanent brain damage may result unless rescued promptly. |